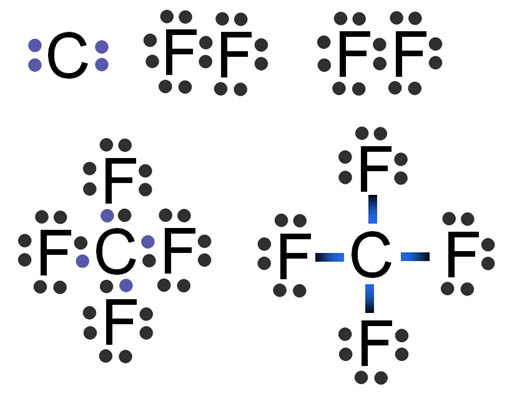
It can also be prepared by the fluorination of carbon dioxide carbon monoxide or phosgene with sulfur tetrafluoride.
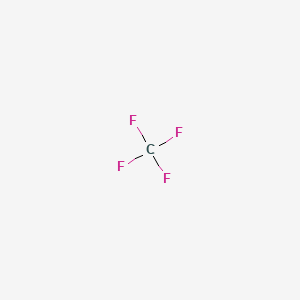
Carbon tetrafluoride state at room temperature.
The initial absorption experiments revealed 165 mg l 1 cf 4 gas dissolved in 10 m naoh.
Because im getting mixed answers from google and cant find it on the msds sheet l.
It was first reported in 1926.
Hg 6 3 p 1 at room temperature and at 300 c.
And hg 6 1 p 1 at room temperature fail to react with carbon tetrafluoride at a measurable rate xe 3 p 1 causes carbon tetrafluoride to decompose with a quantum efficiency of about unity to yield fluorine and an unidentified solid product it is concluded that the energy necessary to break the first c f bond m cf 4 is more than 154 and less.
Although carbon 14 decays into nitrogen 14 through beta decay the amount of carbon 14 in the environment remains constant because new carbon 14 is always being created in the upper atmosphere by cosmic rays.
Is it a liquid or a gas.
Request pdf innovative reductive remediation of carbon tetrafluoride at room temperature by using electrogenerated co1 among the non co2 greenhouse gases carbon tetrafluoride cf4 is the.
With hydrocarbons hydrogen fluoride is a coproduct.
Among the non co 2 greenhouse gases carbon tetrafluoride cf 4 is the most recalcitrant and should be eliminated from the atmosphere in the present study a non combustion electroscrubbing method was used in an attempt to degrade cf 4 with an electrogenerated co 1 mediator in a highly alkaline medium.
At room temperature 25 celcius the carbon tetrachloride is liquid.
Innovative reductive remediation of carbon tetrafluoride at room temperature by using electrogenerated co 1.
Workers that produce or use tetrafluoromethane may breathe in vapors or have direct skin contact.
Tetrafluoromethane is the product when any carbon compound including carbon itself is burned in an atmosphere of fluorine.
Tetrafluoromethane is a colorless odorless gas.
Living things tend to ingest materials that contain carbon so the percentage of carbon 14 within living things is the same as the.
These molecules are polar and can also participate in dipole dipole interactions.
Propanal carbon tetrafluoride has the strongest intermolecular forces because its moloculos can undergo hydrogen bonding interactions which are stronger than dipole dipole interactions and london dispersion forces.
Pu 76 72 c whereas carbon tetrafluoride ce cf4 is in gaseous state at room temperature b p.
It is moderately soluble in water use.
Tetrachloromethane ccl4 is a liquid at romm temp.
It is a by product of aluminum processing.
Author links open overlay panel g.